Structural basis for binding of unsaturated alginate by a carbohydrate-binding module
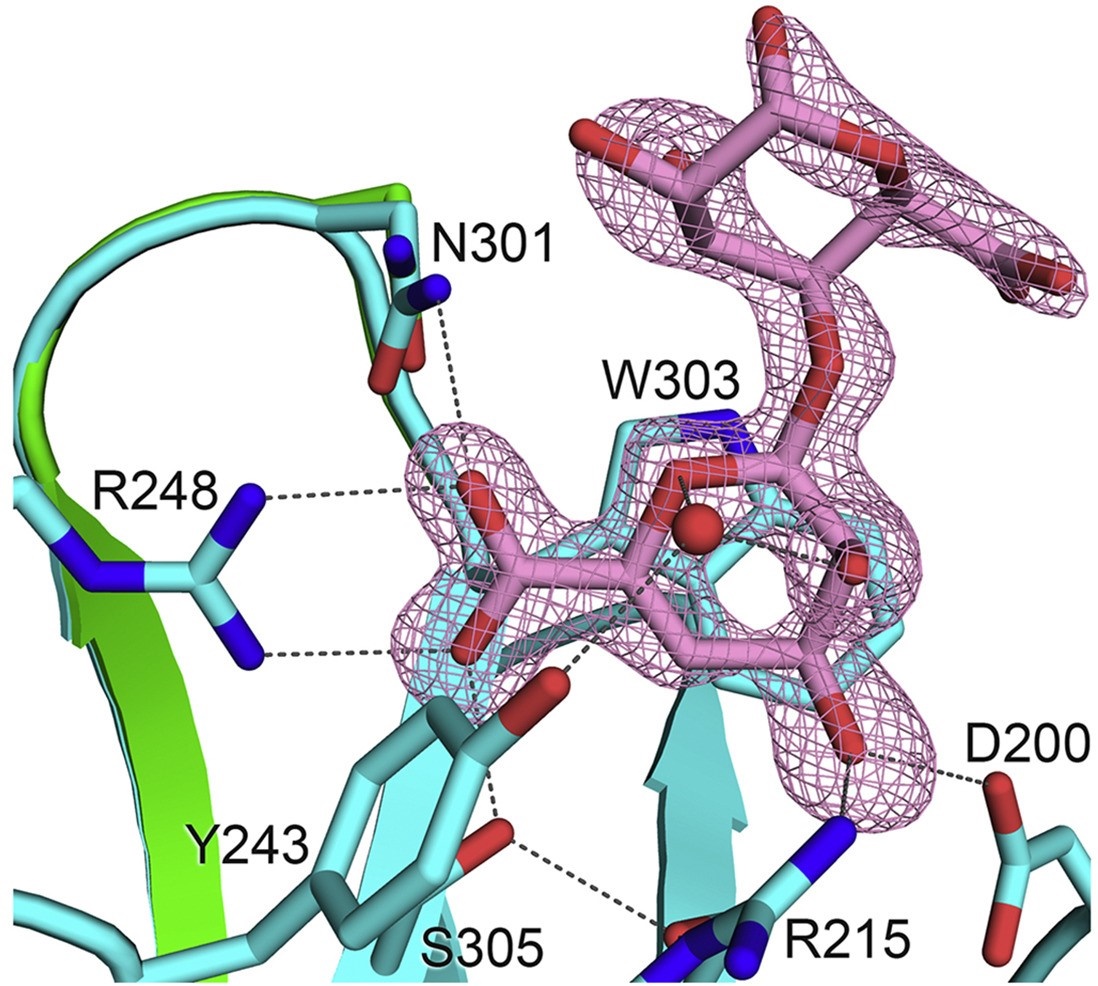
December 2020 — Alginate is a type of negatively charged sugar and is uniquely cleaved by the enzyme alginate lyase into 4,5-unsaturated oligomers, which reportedly have biological activities such as promoting cell growth, stimulating immune responses and even exhibiting antitumour effects. The alginate lyase from the marine bacterium Persicobacter sp. CCB-QB2, AlyQ, contains a domain which belongs to the family 32 carbohydrate-binding modules (CBM32) and binds specifically the unsaturated alginate. Teh Aik Hong and his group have solved the structure of the CBM32 domain in complex with the unsaturated alginate, and identified the key amino acid residues involved in the binding. The complex's structure has also provided an explanation for why the CBM32 domain is unable to bind saturated alginate, which is made up of mannuronic acid and guluronic acid. The structural information further paves the way for utilisation of the CBM32 domain in isolating the useful unsaturated alginate oligomers after enzymatic degradation.
https://www.sciencedirect.com/science/article/pii/S0006291X2031812X
- Hits: 1931