RESEARCH HIGHLIGHTS - Identification of peptide binding sequence of TRIM25 on 14-3-3σ by bioinformatics and biophysical techniques
by De Chen Chianga, Aik-Hong Teh, and Beow Keat Yap
14-3-3σ protein is one of the seven isoforms from the highly conserved eukaryotic 14-3-3 protein family, which function to mediate protein–protein interaction for cell cycle regulation, apoptosis, protein trafficking and signal transduction. Downregulation of 14-3-3σ expression has been observed in various tumours. TRIM25 is responsible for the proteolytic degradation of 14-3-3σ in breast cancer and endometrial cancer, while abrogation of TRIM25 suppresses tumour growth through 14-3-3σ upregulation. As the exact interactions between 14-3-3σ and TRIM25 are unknown, identification of the peptide sequence of TRIM25 that binds 14-3-3σ were thus attempted via both bioinformatics and biophysical techniques. Among five of TRIM25's potential peptide binding sequences predicted from multiple sequence alignments, one peptide was found to bind to 14-3-3σ using nuclear magnetic resonance (NMR) assay (1H CPMG). Competition NMR assays and isothermal titration calorimetry (ITC) suggested that this peptide bound to the amphipathic pocket of 14-3-3σ. Further in silico docking and molecular dynamics simulations showed that it was likely to interact with Lys49, Arg56, Arg129, and Tyr130 of 14-3-3σ. These results suggest that this peptide may serve as a biological probe or as a template for designing novel anticancer agents that disrupt the 14-3-3σ–TRIM25 interaction.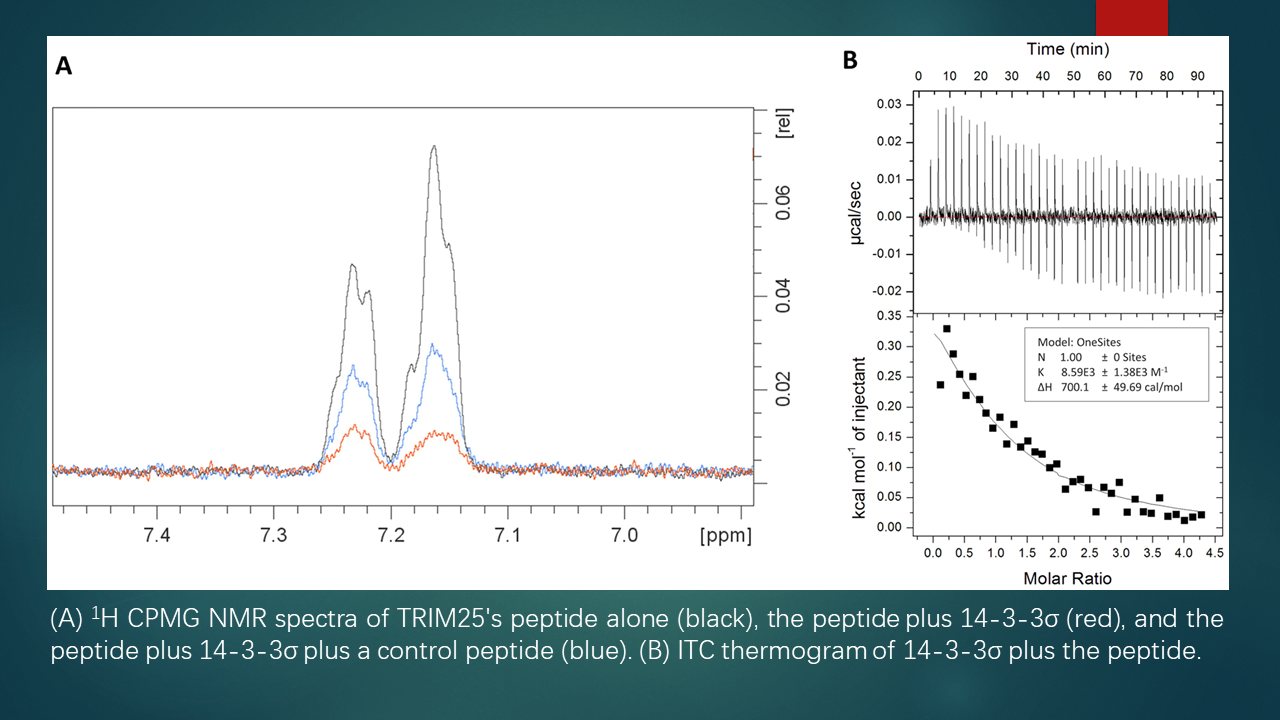
Link: https://www.tandfonline.com/doi/epdf/10.1080/07391102.2023.2172458?needAccess=true&role=button
CCB Ref.: 2023_RH_tah01
Date " 04/07/22023
- Hits: 280